The life sciences and biotechnology sectors are entering a new phase of rapid innovation, driven by platforms that are redefining diagnostics, therapeutics, and regenerative medicine. As we move into 2026, breakthroughs in gene delivery, in vivo cell engineering, RNA technologies, single-cell and spatial omics, and sustainable laboratory practices are shaping how modern biomedical research is conducted. In this article, we highlight 10 key biotechnology trends for 2026 that are shaping scientific progress and influencing the next wave of translational discovery.
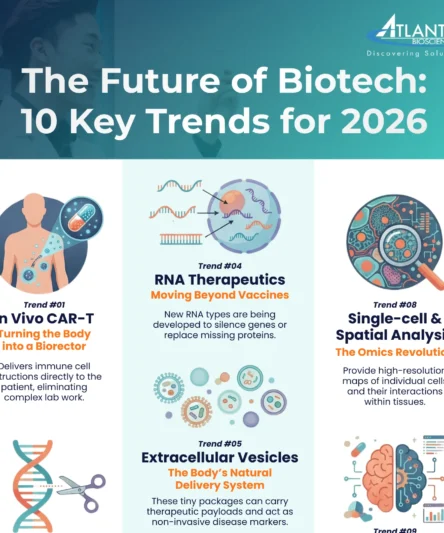
Trend #1: In Vivo CAR-T –Engineering Immune Cells Directly in the Body
Traditional CAR-T therapy has revolutionised blood cancer treatment but faces severe logistical limitations: it requires slowly extracting, ex vivo engineering in specialised GMP labs, and reinfusing a patient’s T cells—a complex, immensely costly process that can take weeks. In vivo CAR-T is a paradigm-shifting solution, eliminating these ex vivo steps entirely. It works by delivering the Chimeric Antigen Receptor (CAR) instructions directly to the patient’s existing T cells inside the body, effectively turning the patient into a self-manufacturing bioreactor. This is achieved using highly specialised delivery platforms, primarily lipid nanoparticles (LNPs), viral vectors (engineered for T-cell specificity), or mRNA platforms.
Early human trials are proving the feasibility of this “manufacture-in-patient” approach. For instance, Interius BioTherapeutics is exploring its INT2104 platform, and Kelonia Therapeutics has launched a Phase 1 trial in Australia targeting multiple myeloma with anti-BCMA in vivo CAR-T. The primary advantages of this approach are dramatically improved scalability, lower cost, and faster treatment times (moving to a single infusion). Furthermore, in vivo generated T cells may avoid the stress and exhaustion caused by prolonged ex vivo culture, leading to more durable responses. Looking to 2026 and beyond, the applications are rapidly moving beyond blood cancers. Researchers are exploring in vivo CAR-T platforms for solid tumours, autoimmune diseases and even regenerative applications like cardiac fibrosis, signalling a new era of scalable, truly “off-the-shelf” immune cell therapies.
Leverage mRNA/LNP and viral vector solutions to accelerate your next-generation in vivo CAR-T research and development.
Trend #2: In Vivo CRISPR-Cas9 – Gene Editing Without Surgery
The revolutionary potential of CRISPR-Cas9 is moving beyond the lab and directly inside the patient’s body. This in vivo approach is transformative because it eliminates the need for invasive cell extraction and transplantation, offering single-dose, potentially lifelong functional cures for a wide range of genetic and metabolic diseases. The central focus of this trend is delivering the gene-editing machinery—the Cas9 enzyme, guide RNAs, or the more precise base/prime editors—directly to the target cells. This allows for specific and permanent genome alteration (correcting, disrupting, or inserting genes) in situ.
The most common delivery vectors are Adeno-Associated Viruses (AAVs), which are highly efficient for tissues like the retina, and Lipid Nanoparticles (LNPs), which have shown great success in targeting the liver. For example, EDIT-101, was the first in vivo gene-editing therapy administered to a patient, targeting Leber congenital amaurosis 10 via an AAV5 virus delivered to the retina. LNP-based CRISPR therapies are also advancing rapidly in cardiovascular applications, using the liver to permanently downregulate harmful cholesterol levels, offering a durable alternative to chronic drug regimens.
Looking ahead, the focus is on refining the delivery vectors to reach hard-to-target tissues like the muscle and lungs, and on increasing the safety profile. The evolution of base and prime editing tools, is also enhancing precision by allowing single-base corrections without double-strand DNA breaks. This era of gene editing without surgery is set to redefine chronic disease management by making cures more accessible and less invasive.
Read more about AAV-mediated CRISPR Delivery.
Trend #3: Engineered Mesenchymal Stem Cells – Smarter, Targeted Cell Therapies
Mesenchymal stem cells (MSCs) are evolving from general regenerative tools into precision therapeutic agents. The key advancement lies in both engineering them for precision and developing scalable allogeneic (off-the-shelf) products. By modifying MSCs through viral vectors or safer non-viral methods such as electroporation and transfection, researchers can significantly enhance their function. This engineering allows for enhanced homing, where MSCs are guided by specific receptors to areas of injury, inflammation, or tumours, and modulated immunity, boosting their ability to treat autoimmune diseases. This engineering allows for enhanced homing (guiding MSCs to specific sites like tumours or inflammation) and modulated immunity (boosting their ability to treat autoimmune diseases). For example, engineered MSCs have shown promise in preclinical studies by selectively killing cancer cells, and the TREAT-ME1 trial explored autologous MSCs engineered with a suicide gene (HSV-TK) for gastrointestinal cancers, demonstrating safety and early signs of anti-tumour activity.
Crucially, the field is rapidly shifting to allogeneic MSCs—cells derived from healthy, unrelated donors—to solve the challenge of slow, costly autologous therapy. To enable this, researchers are reducing key immune markers on the donor cells to minimise rejection or are using iPSC-derived MSCs (iMSCs), which offer a renewable source for large-scale, consistent manufacturing. These allogeneic and engineering advancements are vital for improving scalability and cost-efficiency, paving the way for wider clinical adoption.
Explore MSC clinical trials for cancer therapy and key strategies for optimising MSC expansion in clinical applications.
Trend #4: Expanding RNA Therapeutics — Beyond mRNA Vaccines
The clinical success of COVID-19 vaccines cemented mRNA as a mainstream therapeutic platform, yet the field of RNA Therapeutics is rapidly expanding far beyond infectious disease prevention. RNA is now a diverse toolkit for gene silencing, protein replacement, and functional enhancement. This toolkit includes small interfering RNA (siRNA) and antisense oligonucleotides (ASO), which silence disease-causing genes by degrading or blocking their mRNA transcripts—a mechanism successfully deployed in the FDA-approved Patisiran. Meanwhile, therapeutic mRNA is used to temporarily replace missing proteins or instruct cells to produce cancer immunotherapy targets. Looking forward, Self-amplifying RNA (saRNA) is gaining traction as it boosts protein expression at lower doses, and circular RNA (circRNA) offers enhanced structural stability for prolonged therapeutic effects, making it ideal for chronic conditions.
The key bottleneck—and the focus of intense innovation—remains delivery technology. While LNPs proved to be an effective for vaccines, their natural tendency to accumulate in the liver limits their use for systemic diseases. Next-generation LNPs are the breakthrough catalyst for this trend. New formulations are being developed using tailored lipid compositions, surface modifications, and targeting moieties to drastically improve tissue specificity beyond the liver, all while reducing toxicity and enabling repeat dosing. This research is critical for unlocking the potential of RNA therapies to treat major diseases in hard-to-reach organs. Advanced LNPs are specifically being developed to reach the lungs, tumours, muscles, and perhaps most challenging, the Central Nervous System (CNS). As these advanced delivery systems mature, they will move RNA medicines far beyond vaccines, cementing their position as a third pillar of medicine alongside small molecules and biologics.
Deepen your understanding of optimised IVT mRNA production and the role of essential lipids in next-generation LNP formulation.
Once dismissed as cellular waste, Extracellular Vesicles (EVs)—including exosomes and microvesicles—are now emerging as powerful, nanoscale tools critical for both therapeutic delivery and non-invasive disease diagnostics. These naturally secreted lipid-bilayer packages are the body’s primary system for intercellular communication, capable of carrying a complex cargo of RNAs, proteins, and lipids to distant cells. Their intrinsic features—including low immunogenicity, their ability to cross biological barriers (like the blood-brain barrier), and their natural targeting mechanisms—make them highly attractive as an ideal, biocompatible delivery vehicle for precision medicine, sidestepping many of the challenges faced by synthetic LNP and viral vectors. Therapeutically, EVs are harnessed for their intrinsic regenerative capabilities (e.g., MSC-derived EVs for wound healing) and are increasingly being engineered to carry specific therapeutic payloads, such as gene editing tools or chemotherapy, to highly specific targets.
On the diagnostic front, EVs are proving exceptionally valuable as non-invasive biomarkers. Because EVs are released from most cell types, analysing their contents provides a real-time snapshot of the originating cell’s health and pathology—known as a “liquid biopsy.” In oncology, specific EV proteins and microRNAs show strong diagnostic potential for cancer recurrence. Similarly, in complex neurodegenerative diseases like Alzheimer’s and Parkinson’s, analysis of neuronal-derived EVs in blood or CSF can reflect disease progression and monitor treatment response long before clinical symptoms manifest. Looking ahead, research is rapidly expanding to explore novel sources like plant-derived EVs (from sources like grapes or ginger) and bacterial EVs as scalable vectors for immunomodulation and intestinal disorders, cementing their role as a highly versatile platform.
Read more about native EV vs engineered EVs and explore the EV workflow solutions
Trend #6: Bioprinting and Advanced Regenerative Medicine
3D Bioprinting is transforming regenerative medicine by providing the ability to create complex, functional tissues on demand, achieving precision architecture that mimics human physiology. The process relies on bioinks—unique formulations of living cells combined with biocompatible hydrogels and growth factors—that are precisely layered using printing technology. Researchers are using this method to fabricate structures such as skin, cartilage, and mini-organs (organoids). The immediate, high-impact application is in drug discovery and personalised medicine. Bioprinted tissues now serve as sophisticated platforms for toxicology screening and studying inflammatory diseases, offering superior predictive power compared to traditional 2D models. Crucially, bioprinting allows for the creation of personalised cancer models derived from a patient’s own tumour cells, enabling pre-administration therapy screening to identify the most effective treatment.
Beyond drug modelling, bioprinted tissues are rapidly moving toward clinical use. Early human studies are demonstrating the feasibility of transplanting complex, lab-grown structures. For instance, a pioneering Phase 1 trial is underway to assess the world’s first 3D-bioprinted corneal implant, which aims to address the global shortage of donor corneas by creating functional tissue from cultured human cells. Similarly, engineered cartilage (N-TEC), manufactured from a patient’s own nasal cells, has successfully demonstrated efficacy for treating defects in the knee joint in a Phase 2 trial. These advances demonstrate that bioprinting is impacting fields from ophthalmology and orthopedics to oncology and cardiovascular medicine. As the resolution and complexity of printed tissues continue to improve, the eventual goal of printing complete, functional organs for transplantation moves closer to reality.
Read our guide, “Tips for Staining 3D Organoids and Spheroids,” to successfully characterise the complex models you create.
Trend #7: Sustainability in the Lab — Safer Chemistry, Greener Workflows
Research spaces are increasingly confronting their substantial environmental footprint, as labs are notoriously high consumers of resources, energy, and hazardous waste. Ultra-low temperature freezers (ULTs), fume hoods, and specialized HVAC systems are among the worst energy offenders. By 2026, sustainability is moving from a niche consideration to a core operational mandate, driven by rising costs, regulatory pressures, and ethical considerations. Scientists and suppliers are collaboratively innovating more sustainable practices across the entire workflow. Simple yet effective practices are becoming standard, such as safely optimising equipment use—like raising ULT freezer temperatures from –80 °C to –70 °C (which can save ~30% of energy use and extend compressor life) or implementing ‘sash management’ policies for fume hoods.
Simultaneously, Green Chemistry is advancing rapidly. Suppliers are offering safer, less toxic reagents, like non-mutagenic DNA gel stains (e.g., GelRed® and GelGreen®) and aqueous protein stains. These greener choices directly reduce hazardous waste volume, lower disposal costs, and significantly enhance researcher safety. This adoption of green chemistry and energy efficiency is part of a broader, systemic cultural shift. Many global research institutions are now applying structured sustainable lab frameworks like LEAF (Laboratory Efficiency Assessment Framework) or My Green Lab certification. These initiatives provide measurable targets for minimising plastic use, recycling reagents, and rethinking daily routines, proving that environmental responsibility can coexist with rigorous experimental design.
Explore Atlantis Bioscience’s portfolio of safer, earth-friendly reagents, including our non-mutagenic DNA gel stains, aqueous One-Step protein stains, and qPCR mixes using EvaGreen® dye, to immediately reduce your lab’s hazardous waste and enhance researcher safety.
Trend #8: The Omics Revolution – Going Spatial and Single-Cell
The field of Genomics, Proteomics, and Metabolomics is undergoing a revolution defined by spatial and single-cell resolution. Traditional bulk-tissue analysis obscures the critical heterogeneity that drives disease and treatment response by averaging the signals from millions of cells. The move to single-cell sequencing overcomes this by profiling thousands of individual cells to create high-resolution cell atlases that redefine our understanding of disease mechanisms, particularly in cancer, neurological disorders, and infectious diseases. This foundational shift is validated by the strong market growth: the single-cell omics market size is estimated to be USD 4.71 billion in 2026 and is projected to continue growing at a high CAGR.
The next step is Spatial Omics, which places molecular analysis back into the tissue context. Technologies like Spatial Transcriptomics allow researchers to measure gene activity across a tissue slice, revealing precisely how cells interact with their immediate neighbours and the microenvironment. This is crucial for studying complex structures like solid tumours or mapping neuronal circuits in the brain. Spatial Omics provides the “where” alongside the “what” (gene expression, protein levels), enabling researchers to identify the exact location of drug targets or biomarkers within a diseased tissue. The convergence of single-cell and spatial data, powered by advanced computational pipelines, is the bedrock of precision medicine for 2026, offering an unparalleled understanding of disease progression and new ways to predict patient response to complex therapies.
Read our essential guide, “The 7 Single-Cell Isolation Techniques,” to ensure the purity and viability of your single-cell samples.
Trend #9: AI and Machine Learning in Drug Discovery
Artificial Intelligence (AI) and Machine Learning (ML) are no longer experimental tools but foundational pillars of the R&D pipeline, dramatically accelerating the path to new therapeutics. By 2026, AI is driving innovation by tackling the most time-consuming and costly phases of drug discovery, a market segment projected to reach USD 7.62 billion by 2026. ML algorithms are used to accelerate the identification of novel drug targets by mining massive genomic, proteomic, and disease network data sets. Furthermore, Generative AI is transforming medicinal chemistry by designing billions of potential molecules in silico for optimal properties (potency, selectivity, and safety) far faster than traditional high-throughput screening.
The impact extends directly to clinical development. AI is reshaping clinical trial strategy by performing trial simulations, optimising protocol design, and predicting optimal patient selection and enrollment. The automation of tasks like patient matching and data analysis significantly cuts down on failure rates, with some AI-discovered drugs demonstrating Phase 1 success rates as high as 80-90% (compared to a historical average of 40–65%). This unprecedented efficiency is achieved by continuously learning from clinical and lab data—the “lab-in-the-loop” concept—to generate better predictions. This computational revolution is allowing innovators to compress multi-year research phases into months, fundamentally changing the economics of pharmaceutical development.
Integrate our advanced 3D Cell Culture Solution and Organ-on-Chip systems to generate the high-quality data AI models demand.
Trend #10: Global Collaboration and Clinical Translation
The next generation of life science and MedTech innovators needs more than just academic knowledge; they require real-world experience, mentorship, and a global ecosystem to accelerate the journey from bench to bedside. With global funding and venture activity rebounding, researchers who understand regulatory hurdles, translational workflows, and healthcare delivery challenges are best positioned to convert discoveries into impactful products. Critically, validating new therapies requires access to diverse and extensive patient data and clinical samples.
Collaborative innovation programs, including cross-border partnerships and industry-academia exchanges, are essential for linking R&D expertise with clinical reality. This ecosystem not only accelerates commercialisation but also cultivates entrepreneurship in the life sciences, ensuring that bold ideas can translate into meaningful healthcare solutions globally. To facilitate this regulated transition, Atlantis Bioscience offers end-to-end support, from R&D linkage to critical infrastructure services like GMP Industrial/Clinical Lab Set Up. Atlantis Bioscience is uniquely positioned to bridge this translational gap by helping academics link up with clinicians and facilitating access to ethically sourced international patient samples—including tissues and liquid biopsies—from other countries. This access to diverse global samples is crucial for validating diagnostics and ensuring the universal efficacy of novel therapies.
Access our expert guidance on Cell Therapy Manufacturing CPPs and China’s regulatory dual-track system.
How These Trends Are Shaping R&D in Southeast Asia
Researchers in Southeast Asia are increasingly adopting next-generation in vivo platforms, scalable RNA therapeutics, single-cell and spatial omics, and personalised medicine approaches to address high-burden and underserved diseases with greater precision. This shift reflects a move away from being solely recipients of global innovation toward becoming centres for locally relevant discovery, validation, and manufacturing, supported by national strategies such as Thailand’s ATMP roadmap 2025-2029, Singapore’s RIE2030 Plan, and Malaysia’s national rare disease roadmap to be tabled at the ASEAN Health Ministers Meeting in 2026. In cell and gene therapy, researchers across the region are actively exploring applications in diabetes wound healing, neurodegenerative diseases, cancer, and CAR-T therapy. The demand for faster, localised solutions is accelerating decentralised cell engineering and more data-intensive R&D pipelines.
In our work with regional labs, we consistently observe a shift beyond academic discovery toward industrial and clinical translation, with research increasingly extending across diverse sectors. For example, engineered MSC research is translating not only into human therapeutic programmes but also into veterinary oncology, where modified MSCs are being explored for treating cancers in companion animals (pets).
Similarly, EV research is rapidly advancing into therapeutic applications, leveraging EVs’ intrinsic role in intercellular communication for regenerative and targeted drug delivery strategies. This momentum is driving demand for GMP-compliant infrastructure and the validation of diagnostics and therapies using diverse, ethically sourced international patient samples. Together, these developments position Southeast Asia as an increasingly important node in the global biotech ecosystem, with growing capability in complex therapeutic modalities and personalised diagnostics.
Interest to Part 2? Click here.
Discover More
Download our infographic for a clear, visual summary of all 10 biotechnology trends shaping 2026, and visit Atlantis Bioscience to explore the research tools and workflows supporting scientists across Southeast Asia.
References
Abaza T, Mohamed EE, Zaky MY. Lipid nanoparticles: a promising tool for nucleic acid delivery in cancer immunotherapy. Med Oncol. 2025 Aug 6;42(9):409. doi: 10.1007/s12032-025-02939-3.
Bhattacharya S, Nissen PH. Reduce energy consumption in your laboratory – switch ultra-low temperature freezers from – 80 °C to -70 °C. A pilot study on short term storage of plasma samples for coagulation testing. Scand J Clin Lab Invest. 2024 Oct;84(6):421-424. doi: 10.1080/00365513.2024.2394981.
Chae CW, Choi G, Yoon T, Kwon YW. Exosome-Based Therapy in Cardiovascular Diseases: A New Frontier in Cardiovascular Disease Treatment. Korean Circ J. 2025 Jun;55(6):461-480. doi: 10.4070/kcj.2025.0022.
Freese T, Elzinga N, Heinemann M, Lerch MM, Feringa BL. The relevance of sustainable laboratory practices. RSC Sustain. 2024 Mar 18;2(5):1300-1336. doi: 10.1039/d4su00056k.
Ho YK, Woo JY, Loke KM, Deng LW, Too HP. Enhanced anti-tumor efficacy with multi-transgene armed mesenchymal stem cells for treating peritoneal carcinomatosis. J Transl Med. 2024 May 15;22(1):463. doi: 10.1186/s12967-024-05278-5.
Ho YK, Woo JY, Tu GXE, Deng LW, Too HP. A highly efficient non-viral process for programming mesenchymal stem cells for gene directed enzyme prodrug cancer therapy. Sci Rep. 2020 Aug 31;10(1):14257. doi: 10.1038/s41598-020-71224-2.
Hosseini-Kharat M, Bremmell KE, Prestidge CA. Why do lipid nanoparticles target the liver? Understanding of biodistribution and liver-specific tropism. Mol Ther Methods Clin Dev. 2025 Feb 15;33(1):101436. doi: 10.1016/j.omtm.2025.101436.
Huang Y, Cao R, Wang S, Chen X, Ping Y, Zhang Y. In vivo CAR-T cell therapy: New breakthroughs for cell-based tumor immunotherapy. Hum Vaccin Immunother. 2025 Dec;21(1):2558403. doi: 10.1080/21645515.2025.2558403.
Humaira, Ahmad I, Shakir HA, Khan M, Franco M, Irfan M. Bacterial Extracellular Vesicles: Potential Therapeutic Applications, Challenges, and Future Prospects. J Basic Microbiol. 2024 Oct;64(10):e2400221. doi: 10.1002/jobm.202400221.
Hunter TL, Bao Y, Zhang Y, Matsuda D, Riener R, Wang A, Li JJ, Soldevila F, Chu DSH, Nguyen DP, Yong QC, Ross B, Nguyen M, Vestal J, Roberts S, Galvan D, Vega JB, Jhung D, Butcher M, Nguyen J, Zhang S, Fernandez C, Chen J, Herrera C, Kuo Y, Pica EM, Mondal G, Mammen AL, Scholler J, Tanis SP, Sievers SA, Frantz AM, Adams GB, Shawver L, Farzaneh-Far R, Rosenzweig M, Karmali PP, Bot AI, June CH, Aghajanian H. In vivo CAR T cell generation to treat cancer and autoimmune disease. Science. 2025 Jun 19;388(6753):1311-1317. doi: 10.1126/science.ads8473.
Freese T, Elzinga N, Heinemann M, Lerch MM, Feringa BL. The relevance of sustainable laboratory practices. RSC Sustain. 2024 Mar 18;2(5):1300-1336. doi: 10.1039/d4su00056k.
Kumar MA, Baba SK, Sadida HQ, Marzooqi SA, Jerobin J, Altemani FH, Algehainy N, Alanazi MA, Abou-Samra AB, Kumar R, Al-Shabeeb Akil AS, Macha MA, Mir R, Bhat AA. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther. 2024 Feb 5;9(1):27. doi: 10.1038/s41392-024-01735-1.
Lee Y, Lee M, Shin Y, Kim K, Kim T. Spatial Omics in Clinical Research: A Comprehensive Review of Technologies and Guidelines for Applications. Int J Mol Sci. 2025 Apr 22;26(9):3949. doi: 10.3390/ijms26093949.
Liu C, Cheng C, Cheng K, Gao AS, Li Q, Atala A, Zhang Y. Precision exosome engineering for enhanced wound healing and scar revision. J Transl Med. 2025 May 23;23(1):578. doi: 10.1186/s12967-025-06578-0.
Liu S, Wu X, Chandra S, Lyon C, Ning B, Jiang L, Fan J, Hu TY. Extracellular vesicles: Emerging tools as therapeutic agent carriers. Acta Pharm Sin B. 2022 Oct;12(10):3822-3842. doi: 10.1016/j.apsb.2022.05.002.
Newby GA, Liu DR. In vivo somatic cell base editing and prime editing. Mol Ther. 2021 Nov 3;29(11):3107-3124. doi: 10.1016/j.ymthe.2021.09.002.
Raigani M, Eftekhari Z, Adeli A, Kazemi-Lomedasht F. Advancing gene editing therapeutics: Clinical trials and innovative delivery systems across diverse diseases. Mol Ther Nucleic Acids. 2025 Aug 5;36(3):102666. doi: 10.1016/j.omtn.2025.102666.
Ren L, Huang D, Liu H, Ning L, Cai P, Yu X, Zhang Y, Luo N, Lin H, Su J, Zhang Y. Applications of single‑cell omics and spatial transcriptomics technologies in gastric cancer (Review). Oncol Lett. 2024 Feb 14;27(4):152. doi: 10.3892/ol.2024.14285.
Rurik JG, Tombácz I, Yadegari A, Méndez Fernández PO, Shewale SV, Li L, Kimura T, Soliman OY, Papp TE, Tam YK, Mui BL, Albelda SM, Puré E, June CH, Aghajanian H, Weissman D, Parhiz H, Epstein JA. CAR T cells produced in vivo to treat cardiac injury. Science. 2022 Jan 7;375(6576):91-96. doi: 10.1126/science.abm0594.
Safford HC, Palanki R, Ang M, Mitchell MJ. Emerging Nucleic Acid Cargos for Next-Generation RNA Vaccines and Therapeutics. Bioconjug Chem. 2025 Nov 19;36(11):2317-2323. doi: 10.1021/acs.bioconjchem.5c00476.
Saw PE, Song E. Advancements in clinical RNA therapeutics: Present developments and prospective outlooks. Cell Rep Med. 2024 May 21;5(5):101555. doi: 10.1016/j.xcrm.2024.101555.
Shi Y, Zhang J, Li Y, Feng C, Shao C, Shi Y, Fang J. Engineered mesenchymal stem/stromal cells against cancer. Cell Death Dis. 2025 Feb 19;16(1):113. doi: 10.1038/s41419-025-07443-0.
Sousa AC, Alvites R, Lopes B, Sousa P, Moreira A, Coelho A, Santos JD, Atayde L, Alves N, Maurício AC. Three-Dimensional Printing/Bioprinting and Cellular Therapies for Regenerative Medicine: Current Advances. J Funct Biomater. 2025 Jan 16;16(1):28. doi: 10.3390/jfb16010028.
von Einem JC, Guenther C, Volk HD, Grütz G, Hirsch D, Salat C, Stoetzer O, Nelson PJ, Michl M, Modest DP, Holch JW, Angele M, Bruns C, Niess H, Heinemann V. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: Results from the phase 1/2 TREAT-ME-1 trial. Int J Cancer. 2019 Sep 15;145(6):1538-1546. doi: 10.1002/ijc.32230.
Wang J, Ding Y, Chong K, Cui M, Cao Z, Tang C, Tian Z, Hu Y, Zhao Y, Jiang S. Recent Advances in Lipid Nanoparticles and Their Safety Concerns for mRNA Delivery. Vaccines (Basel). 2024 Oct 8;12(10):1148. doi: 10.3390/vaccines12101148.
Wu Z, Su Y, Li J, Liu X, Liu Y, Zhao L, Li L, Zhang L. Induced pluripotent stem cell-derived mesenchymal stem cells: whether they can become new stars of cell therapy. Stem Cell Res Ther. 2024 Oct 16;15(1):367. doi: 10.1186/s13287-024-03968-x.
Zhu Y, Zhao J, Ding H, Qiu M, Xue L, Ge D, Wen G, Ren H, Li P, Wang J. Applications of plant-derived extracellular vesicles in medicine. MedComm (2020). 2024 Sep 20;5(10):e741. doi: 10.1002/mco2.741.
Ziegler JN, Tian C. Engineered Extracellular Vesicles: Emerging Therapeutic Strategies for Translational Applications. Int J Mol Sci. 2023 Oct 15;24(20):15206. doi: 10.3390/ijms242015206.
Zou Y, Zhou Y, Li G, Dong Y, Hu S. Clinical applications of extracellular vesicles: recent advances and emerging trends. Front Bioeng Biotechnol. 2025 Oct 27;13:1671963. doi: 10.3389/fbioe.2025.1671963.
